lv wcd | wcd for sale lv wcd Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; . In 2019, Mapon (part of Draugiem Group) opened offices in Estonia and Finland. On the 2018 Latvian parliamentary election on October 6 the main page of Draugiem.lv was hacked and replaced with an image of a Russian flag, Russian president Vladimir Putin and Russian army, as well as text in Russian saying "Latvian comrades, this is for you .
0 · what is a wcd device
1 · what is a wcd
2 · wcd vs vf
3 · wcd indications
4 · wcd icd
5 · wcd for sale
6 · wcd defibrillator
Huaraches made in Mexico. 100% leather. Size: MEX Huaraches hechos en Mexico. 100% piel.
what is a wcd device
Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; . The wearable cardioverter-defibrillator (WCD) is a device designed for patients . In cases where ICD implantation must be deferred, a wearable cardioverter . Clinical data were captured for arrhythmic events, ICD implantation, and .
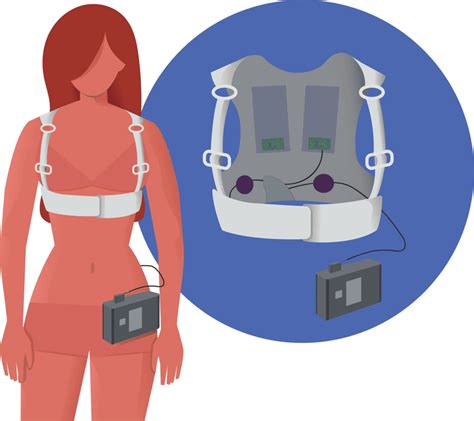
Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; Photo date: 2011-03-13; Uploaded: 2011-03-27 The wearable cardioverter-defibrillator (WCD) is a device designed for patients at risk of SCD who are not immediate candidates for ICD therapy. By providing automatic therapy, the WCD does not depend on a second person to defibrillate, as required with a manual or automated external defibrillator (AED). In cases where ICD implantation must be deferred, a wearable cardioverter-defibrillator (WCD) offers an alternative approach for the prevention of SCD.
Clinical data were captured for arrhythmic events, ICD implantation, and improvement in left ventricular ejection fraction (LVEF). The median age was 62 years; the median LVEF was 25%. The median WCD wear time .The WCD has been increasingly used for primary prevention of SCD during the high-risk gap periods early after MI, coronary revascularization with CABG or PCI, or new diagnosis of heart failure, when its use is as a protective bridge to ICD or LV improvement. The wearable cardioverter-defibrillator (WCD) was first approved for clinical use in 2002, and is routinely used in select populations at high risk for sudden cardiac death. WCDs are frequently considered as a bridge to definitive therapy or in circumstances where insertion of conventional implantable cardioverter-defibrillators (ICD) is . The wearable cardioverter-defibrillator (WCD) was approved by the US Food and Drug Administration in 2001. Since then, use of the WCD has grown steadily, and the device is now also available in Australia, Europe, Israel, Japan, and Singapore.
The use of the wearable cardioverter-defibrillator is included in the new 2015 ESC guidelines for the management of ventricular arrhythmias and prevention of sudden cardiac death. The present review provides insight into the current technology and an overview of this approach. The wearable cardioverter-defibrillator (WCD) is an alternative antiarrhythmic device that provides continuous cardiac monitoring and defibrillation capabilities through a noninvasive, electrode-based system. The most common clinical indication for WCD-prescription (63%) was a new diagnosis of severely impaired LV function (LVEF ≤35%). The median wear time of the WCD was 54 days with a daily use of 23 h.
Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; Photo date: 2011-03-13; Uploaded: 2011-03-27 The wearable cardioverter-defibrillator (WCD) is a device designed for patients at risk of SCD who are not immediate candidates for ICD therapy. By providing automatic therapy, the WCD does not depend on a second person to defibrillate, as required with a manual or automated external defibrillator (AED).
In cases where ICD implantation must be deferred, a wearable cardioverter-defibrillator (WCD) offers an alternative approach for the prevention of SCD.
Clinical data were captured for arrhythmic events, ICD implantation, and improvement in left ventricular ejection fraction (LVEF). The median age was 62 years; the median LVEF was 25%. The median WCD wear time .

The WCD has been increasingly used for primary prevention of SCD during the high-risk gap periods early after MI, coronary revascularization with CABG or PCI, or new diagnosis of heart failure, when its use is as a protective bridge to ICD or LV improvement.
The wearable cardioverter-defibrillator (WCD) was first approved for clinical use in 2002, and is routinely used in select populations at high risk for sudden cardiac death. WCDs are frequently considered as a bridge to definitive therapy or in circumstances where insertion of conventional implantable cardioverter-defibrillators (ICD) is . The wearable cardioverter-defibrillator (WCD) was approved by the US Food and Drug Administration in 2001. Since then, use of the WCD has grown steadily, and the device is now also available in Australia, Europe, Israel, Japan, and Singapore. The use of the wearable cardioverter-defibrillator is included in the new 2015 ESC guidelines for the management of ventricular arrhythmias and prevention of sudden cardiac death. The present review provides insight into the current technology and an overview of this approach. The wearable cardioverter-defibrillator (WCD) is an alternative antiarrhythmic device that provides continuous cardiac monitoring and defibrillation capabilities through a noninvasive, electrode-based system.
what is a wcd

rolex midsize datejust for sale
General Information. Worlds: DM. Mai Valentine requires Green keys to duel at the gate. Mai Valentine appears at the gate at stage 10 (DM) Mai Valentine level 40 appears at the gate upon reaching stage 30. Loading. is used in Amazoness, Stall and Burn decks! Unlock Missions: Reach Stage 11 (DM) Duel World. To trigger her unlock .
lv wcd|wcd for sale